Project
One of the most important paradigms of heterogeneous catalysis is the concept of "supported catalyst", consisting of an active phase (typically small metal particles) dispersed on a support (usually an inorganic oxide). In some cases, such as Au/TiO2, interfacial sites between the metal and the support are invoked as essential to the catalytic activity.
While the working principle is very different, photocatalysis shares the same assumption that at least some active sites are located on metallic nanoparticles (NPs) supported on a semiconductor.
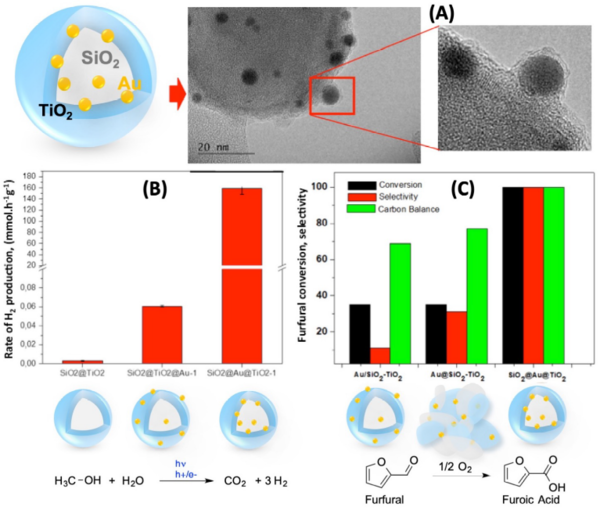
Using a core-shell architecture, Au NPs were sandwiched between a SiO2 core and a TiO2 shell of a few nanometres, denominated herein as “SiO2@Au@TiO2” (Figure 1A). Such a concept of nanoparticle’s coating (but with a larger coating thickness of a few dozens of nanometres) was previously proposed to boost photocatalytic performances by increasing the contact surface between the co-catalyst and the semiconductor. It was also applied to prevent leaching in heterogeneous catalysis, but at the expense of activity since metallic sites are barely exposed to the surface. Using the aforementioned SiO2@Au@TiO2 system with an especially thin TiO2 over-layer thanks to a specific methodology developed at LCP, we observed a tremendous enhancement surprisingly in both photocatalytic and catalytic performances compared to those of a conventional homologous Au/TiO2 catalyst: Indeed, both (i) the photocatalytic production of H2 in the presence of a methanolic solution (performed at LCP; Figure 1B) and the liquid phase oxidation of furfural to furoic acid with impressive selectivity (FA) using O2 as an oxidant (performed at UCCS; Figure 1C) were significantly higher than those of conventional catalytic systems. The main objective of INGENCat is then to provide fundamental understanding of such novel highly active core-shell catalysts for furfural valorization. We intend to identify the active sites and unravel the associated reaction mechanisms related to the oxidation of furfural by means of catalytic and photocatalytic oxidation which will ultimately enable back-optimization of the system to propose ultraefficient catalysts.